The pharma-biotech sector in the face of the COVID-19 crisis: first lessons learned
The pandemic caused by COVID-19 has been generated in an extremely short time. Since the first case recognized by the WHO, on December 8, 2019, and in approximately three months, 157,648 people have been infected and 5,746 have died(as of March 15). In our country, since the confirmation of the first infection in La Gomera on February 1, in just a month and a half we have reached 7,700 infected and approximately 300 deaths. This spread profile , as well as the deleterious effects on some population groups, has forced the adoption of severe measures of confinement of the population.
However, in some countries, the possibility is being considered of implementing more lax confinement measures and thus favoring the development of the so-called immunity of group to accelerate the disappearance of the infectious agent, as is the case in the United Kingdom. This strategy would be more beneficial in minimizing the economic effects of the pandemic, but according to experts in England, it will lead to the collapse of hospitals and a high cost in human lives.
As they say, in the midst of every crisis lies the seed of new opportunities. Well, from the point of view of management pharmaceuticals, we could highlight 3 areas in which this crisis can provide us with lessons that will help us to face future health crises:
1. The first has to do with the medical devices necessary for the correct development of work of staff involved in the care of patients infected by coronavirus. Fundamentally, these medical devices include protective elements (masks, gloves, gowns, etc.) and special life support equipment such as artificial respirators. In general, these items are being supplied normally in healthcare centers, although there have been occasional cases of stock-outs, which could become generalized in the near future. This crisis has highlighted the lack of local manufacturers at Spanish and European level of this subject of materials which, as we are seeing, are strategic for the European Union. The Spanish Government, through the Ministry of Health, has centralized the purchase of these supplies, but there are already evident demands for more material from healthcare professionals and some autonomous communities. The manufacture of this medical device subject is not as simple as one might think, since all medical devices must go through a regulatory approval and clinical validation process. The definition of medical device includes, in a simplified way, all products used in the attendance healthcare that are not drugs, of very different nature and purpose. This category of products includes all these protective materials as well as in vitro diagnostic products. They are therefore a category of products of vital importance in this crisis; in fact, at the present time, they are more strategic than the potential drugs or vaccines that are sure to be developed in the medium term deadline.
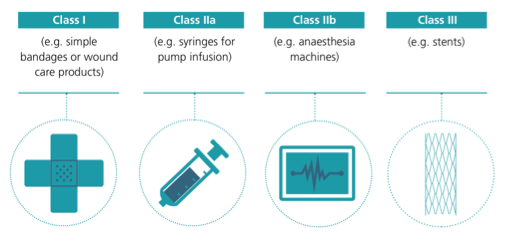
Figure 1. Classification of medical devices (MedTech Europe; 2017/745/EU).
It is important to understand that these products, precisely because they are so important in ensuring the safety of physicians and professionals, must meet certain quality and regulatory requirements. For this reason, approval procedures are longer and more complex than might be thought a priori from public opinion. Medical devices (Figure 1) are regulated by harmonized health regulations in the European Union. The manufacturer, whether European or not, who wants to market one of these medical devices in Europe, approaches a European assessment body, the so-called notified bodies, submitting documentation on the design, manufacturing and sterilization processes, performance tests, clinical trials, packaging materials, the technical standards they comply with and the information accompanying the product. The notified body evaluates this documentation and conducts an audit at the facility where the product is manufactured. If the result of the verifications is favorable, it issues a certificate of conformity that allows to place the issue of notified organism together with the distinctive CE in the product, which indicates that it fulfills the requirements of the regulation. With this marking, the product can be marketed in all European Union countries without the need for further assessment. It can be understood that in this crisis we depend on manufacturers who already have regulatory approval for the manufacture of this subject of products and that it is neither simple nor immediate to put new factories to work.
Figure 2 sample issue of employees in health technology companies (MedTEch) per 10,000 inhabitants in different European countries. Spain is one of the countries with the least employment in this area and is a net importer of medical devices, so it will be strategic to promote the creation of companies in this health sector area (we should bear in mind that 95% of MedTech companies are small and medium-sized companies). FENIN (Spanish Federation of Healthcare Technology Companies) currently has 65 companies registered in Spain that manufacture, import and distribute medical devices designed and intended for one-time use.
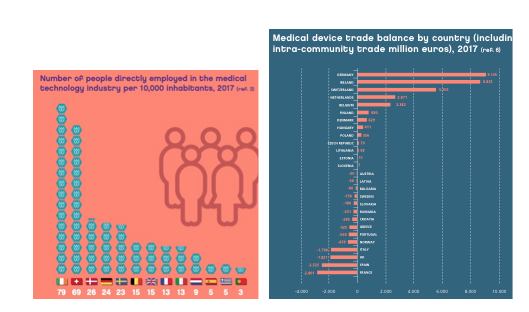
People employed in the medical devices industry in 2017 (expressed in issue of people per 10000 inhabitants). Trade balance in medical devices by country.
2. A second aspect relates to COVID-19 diagnostic systems. On this point, it must be acknowledged that the Spanish biopharmaceutical industry has been a pioneer and has been quick to place several COVID-19 diagnostic kits on the market, which are already being used in our country and in different European countries. Diagnostic products are also medical devices, which can be classified into 4 different types (Figure 3).
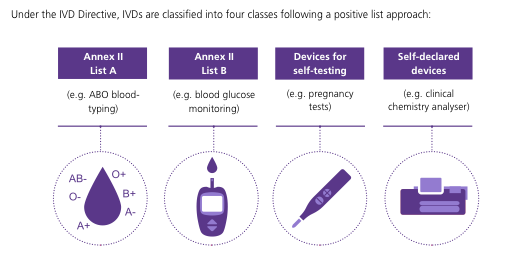
Figure 3. Classification of diagnostic products (IVD Directive; 2017/746/EU).
The Spanish business Genomica, of group Pharmamar, commercialized on March 6 two diagnostic tests for COVID-19 clinically validated on patient samples at partnership with high school Carlos III of Madrid, as required by the health authorities. This has been made possible by the availability of 21 diagnostic tests for respiratory viruses, including 3 coronaviruses (HCoV-229E, HCoV-OC43 and HCoV-NL63) at business . This previous knowledge has been instrumental in the rapid development of a diagnostic test for the new conoravirus. Although the development of a diagnostic test is more complex than that of design and approval of disposable materials, Spain has been able to offer validated products in record time by having the knowledge and the scientific and industrial equipment.
Another Spanish company that has developed diagnostic tests for COVID-19 is CerTest Biotech, a business that collaborates with the University of Navarra, at partnership with business BD (Becton, Dickinson and Company). The test is based on real-time polymerase chain reaction (PCR), identifying and amplifying a specific sequence of the virus DNA in clinical samples. This test marketed by BD in Europe (not available for the United States) will work on the diagnostic platforms already installed in hospitals. It is important to remember that the clinical impact of having these diagnostic tests available is immense, allowing physicians to quickly and reliably identify patients with COVID-19 and proceed with their isolation and appropriate treatment, as well as certifying patients who have overcome the disease. To put this achievement in perspective, on March 9, Anthony Fauci (director of the National Institutes of Health's National Institute of Allergy and Infectious Diseases) warned in the journal JAMA that the U.S. needed to accelerate the pace to get more than one million diagnostic kits for COVID-19 in the next two weeks.
3. The third aspect, and perhaps the most important in the medium term deadline, refers to the therapeutic treatment of the infection. Unfortunately, it is in this aspect core topic where the status has considerable room for improvement. At present, the pharmacological treatment patients receive is purely symptomatic (e.g., paracetamol). In certain hospitals, in severe cases, patients are being treated with available antivirals, although the results are disappointing. It is true that many laboratories around the world are working against the clock to develop new drugs or an effective vaccine against COVID-19, development .
programs of study At present, worldwide, there are about 70 clinical trials under way for the treatment of COVID-19. Of these programs of study 46 are testing drug treatments, including mesenchymal stem cell therapies, antivirals (mostly a combination of lopinavir and ritonavir, together with interferon) and vaccines. In addition, several laboratories are also launching research to develop vaccines and treatments that could soon start clinical trials at development . However, it is important to remember that these treatments will take (at best) 12 to 18 months to reach the market as it is critical to validate the efficacy and safety of these treatments before administering them to patients on the clinical internship . There are several reasons to explain this time lag between the current pressing need and the potential solution months/years away. Firstly, a new drug to be put on the market has to comply with some requirements efficacy and safety requirements that can be demonstrated by clinical trials, in addition to some requirements quality requirements in terms of its industrial manufacture, which substantially increase (in comparison with other high-tech products) its development. A second aspect has to do with the zoonotic origin (it passed from an animal to a human host) of the pathogen. This does not facilitate a rapid and effective response, as its mechanism of action and behavior are still unknown.
Obviously these reasons should not be excuses to draw lessons and apply solutions that could mitigate the next epidemics. COVID-19 is not the first pathogen (nor will it be the last) to produce a pandemic, which is inevitable in an interconnected world. Today the global issue of daily deaths from novoviruses is 10 times higher than from coronaviruses. In the case of rotavirus deaths, the number of daily deaths is 20 times higher. It is also important to bear in mind that the availability of a treatment or vaccine is not a panacea. Thus, for example, the healthcare burden figures associated with seasonal influenza in Spain in the 2018-2019 campaign are very significant: 490,000 mild cases in primary care, 35,300 hospitalizations, 2,500 ICU admissions and 6,300 deaths. All this with the vaccination of 54% of those over 65 years of age, which prevented, in that age group , 40% of admissions to ICU and deaths.
Of course, these health emergencies occur mostly in low- and middle-income countries. We thought we were protected against this subject of misfortunes and the current crisis shows us that this is not the case. In recent decades, we have neglected the basic research in biology and immunology, as well as the development of anti-infectives and vaccines. It is disappointing to note that there are no rapid and effective procedures to develop vaccines and that the issue of adjuvants (immune enhancers or modulators) approved for use in humans is extremely low. We also need to enhance the manufacturing and stocking systems of strategic health products for use and protection of staff in this subject pandemic. We also need early warning and rapid response systems and protocols to enable effective containment systems. And, above all, we need to accept that our "developed" societies do not live in isolation in a globalized world and that they are vulnerable to many factors beyond our control.
Esperanza Regueras and Juan M. Irache
Master's Degree in Pharma-Biotech Company Management
University of Navarra
