An error occurred while processing the template.
The following has evaluated to null or missing:
==> CheckImagenFieldSet.CheckImagenFieldSetFieldSet.ImagenTexto [in template "10136#10174#3653718" at line 78, column 82]
----
Tip: It's the step after the last dot that caused this error, not those before it.
----
Tip: If the failing expression is known to legally refer to something that's sometimes null or missing, either specify a default value like myOptionalVar!myDefault, or use <#if myOptionalVar??>when-present<#else>when-missing</#if>. (These only cover the last step of the expression; to cover the whole expression, use parenthesis: (myOptionalVar.foo)!myDefault, (myOptionalVar.foo)??
----
----
FTL stack trace ("~" means nesting-related):
- Failed at: ${CheckImagenFieldSet.CheckImagenFiel... [in template "10136#10174#3653718" at line 78, column 80]
----
1<!-- AQUI CARGAMOS LA HOJA DE ESTILOS -->
2
3 <#setting locale="es_ES">
4 <!-- FUNCION JAVASCRIPT QUE MUESTRA Y OCULTA EL REPRODUCTOR DE SONIDO -->
5 <script type="text/javascript">d
6function show(num) {
7obj = document.getElementById('player' + num);
8obj.style.display = (obj.style.display=='none') ? 'block' : 'none';
9}
10function openWindow(url) {
11 window.open(url, "new", "directories=no, location=no, menubar=no, scrollbars=yes, statusbar=no, tittlebar=no, width=800, height=800");
12}
13function viewDetail(nameAuthor){
14/*v1.0 before friendly URLs...*/
15/*
16var url = location.href;
17var asset = "";
18if(url.indexOf("/-/")!= -1){
19url = location.href.split("/-/")[0];
20asset = "/-/"+location.href.split("/-/")[1];
21
22}
23url = url.split("/");
24location.href = location.href.replace(asset,"").replace(url[url[url.length-1],"")+"news-author?nameAuthor="+nameAuthor;
25*/
26/*V1.1*/
27var header = "";
28 if (location.href.indexOf("https://")== -1){
29var url = location.href.replace("http://","");
30header = "https://";
31}else{
32var url = location.href.replace("https://","");
33header = "https://";
34}
35var urlSplt = url.split("/");
36if((url.indexOf("/en/")== -1) && (url.indexOf("/en/")== -1)){
37//alert("has not");
38url = urlSplt[0]+"/"+urlSplt[1]+"/"+urlSplt[2]+"/news-author?nameAuthor="+nameAuthor;
39
40}else{
41url = urlSplt[0]+"/"+urlSplt[1]+"/"+urlSplt[2]+"/"+urlSplt[3]+"/news-author?nameAuthor="+nameAuthor;
42}
43location.href= header+url;
44return false;
45}
46 </script>
47
48
49<div id="mainNoticia" class="mainNoticia newsV2">
50
51 <!-- TÍTULOS -->
52 <div class="titulos">
53 <h2><#if Titulo_noticia.getData()??>${Titulo_noticia.getData()}</#if></h2>
54 <script type="text/javascript">
55 $(document).ready(function () {
56document.title = "${htmlUtil.escapeJS(Titulo_noticia.getData()?default(''))}";
57
58 if ($('.breadcrumbs li.last span').length) {
59 $('.breadcrumbs li.last span').text('$htmlUtil.escapeJS($News_title.getData())');
60 }
61 });
62 </script>
63 <p class="subtitulo"><strong><#if Entradilla.getData()??>${Entradilla.getData()}</#if></strong></p>
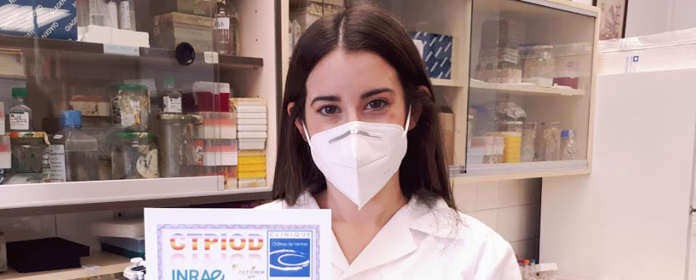
64 </div>
65
66
67 <!-- IMAGEN O VIDEO -->
68 <div class="videoOimagen">
69 <div class="videoOimagen-inner">
70
71 <#if getterUtil.getBoolean(CheckImagenFieldSet.CheckImagen.getData())>
72
73 <div class="imagen">
74
75 <#if CheckImagenFieldSet.CheckImagenFieldSetFieldSet.Imagen.getData()?has_content>
76 <img class="foto" src="${CheckImagenFieldSet.CheckImagenFieldSetFieldSet.Imagen.getData()}" alt="Descripcion de la imagen"/>
77 <#else>
78 <img class="foto" src="${CheckImagenFieldSet.CheckImagenFieldSetFieldSet.ImagenTexto.getData()}" alt="Descripcion de la imagen"/>
79 </#if>
80 <#if validator.isNotNull(CheckImagenFieldSet.CheckImagenFieldSetFieldSet.Texto_imagen.data) || validator.isNotNull(CheckImagenFieldSet.CheckImagenFieldSetFieldSet.Autor_imagen.data)>
81 <div class="textoFoto">
82 <span> ${htmlUtil.unescape(CheckImagenFieldSet.CheckImagenFieldSetFieldSet.Texto_imagen.getData())}</span>
83 <span class="autor">FOTO: ${CheckImagenFieldSet.CheckImagenFieldSetFieldSet.Autor_imagen.getData()}</span>
84 </div>
85 </#if>
86 </div>
87</#if>
88
89 <!-- VIDEOS -->
90 <#list CheckVideo.getSiblings() as video>
91 <#if getterUtil.getBoolean(CheckVideoFieldSet.CheckVideo.getData())>
92 <#if (CheckVideoFieldSet.CheckVideoFieldSetFieldSet.Video_Youtube.getData()?? || CheckVideoFieldSet.CheckVideoFieldSetFieldSet.Video_Biblioteca.getData()??)>
93 <div class="video">
94 <#if CheckVideoFieldSet.CheckVideoFieldSetFieldSet.Video_Biblioteca.getData()?has_content>
95 <video controls style="height:355px;width:100%;">
96 <source src="$video.Video_Biblioteca.data" type="video/mp4"/>
97 <source src="$video.Video_Biblioteca.data" type="video/ogg"/>
98 Your browser does not support the video tag.
99 </video>
100 <#else>
101 <iframe style="height:355px;width:100%;" frameborder="0" allowfullscreen></iframe>
102 <script>
103 $(document).ready(function () {
104
105
106 var url = ' ${CheckVideoFieldSet.CheckVideoFieldSetFieldSet.Video_Youtube.getData()}';
107 var id = url.split(/(?:youtu\.be\/|v\/|embed\/|watch\?v=)/)[1].split("&")[0];
108 $("iframe").attr("src",youtube+ id);
109 });
110 </script>
111 </#if>
112
113 <#if validator.isNotNull(video.Titulo_video.getData())>
114 <div class="titulo"><strong>$video.Titulo_video.getData()</strong></div>
115 </#if>
116 </div>
117 </#if>
118 </#if>
119 </#list>
120 </div>
121 </div>
122
123 <!-- BLOQUE PRINCIPAL -->
124 <div class="leftNoticia">
125 <!-- Aquí se inserta el portlet -->
126 <div class="artRecurInteres"></div>
127 <script>
128 $(document).ready(function () {
129 var portlet = $('#p_p_id_ArticleResourcesInteres_WAR_ArticleResourcesInteresportlet_');
130 if (portlet.length) {
131 portlet.appendTo('.artRecurInteres');
132 var stars = portlet.find('.taglib-ratings.stars');
133 if (stars.length) {
134 portlet.find('.liferay-rating-score').insertBefore('.liferay-rating-vote');
135 }
136 }
137 });
138 </script>
139 </div>
140
141 <!-- DERECHA NOTICIA -->
142 <div class="rightNoticia">
143 <div class="dateNameNew">
144<#assign rawFecha = .vars['reserved-article-modified-date'].data?string>
145<#assign fechaParsed = rawFecha?date("EEE, dd MMM yyyy HH:mm:ss Z")>
146<span class="fechaNoticia">
147 ${fechaParsed?string("dd/MM/yy HH:mm")}
148</span>
149 <#list Nombre_autor.getSiblings() as autor>
150 <a href="#" onclick="verDetalle('$autor.data')" class="nombreAutor">${autor.getData()}</a>
151 </#list>
152 </div>
153
154
155 <#if (Cuerpo1FieldSet.Cuerpo1.getData())??>
156${{Body1FieldSet.Body1.getData()}
157</#if>
158
159<#if (Cuerpo2FieldSet.Cuerpo2FieldSetFieldSet.ladilloRev1.getData())??>
160 <span class="ladillo"> ${Cuerpo2FieldSet.Cuerpo2FieldSetFieldSet.ladilloRev1.getData()}</span>
161</#if>
162
163<#if (Cuerpo2FieldSet.Cuerpo2.getData())??>
164${Body2FieldSet.Body2.getData()}
165</#if>
166
167<#if (Cuerpo3FieldSet.Cuerpo3FieldSetFieldSet.ladilloRev2.getData())??>
168 <span class="laddillo"> ${Cuerpo3FieldSet.Cuerpo3FieldSetFieldSet.ladilloRev2.getData()} </span>
169</#if>
170
171<#if (Cuerpo3FieldSet.Cuerpo3.getData())??>
172${{Body3FieldSet.Body3.getData()}
173</#if>
174
175<#if (Cuerpo4FieldSet.Cuerpo4FieldSetFieldSet.ladilloRev3.getData())??>
176 <span class="ladillo"> ${Cuerpo4FieldSet.Cuerpo4FieldSetFieldSet.ladilloRev3.getData()} </span>
177</#if>
178
179<#if (Cuerpo4FieldSet.Cuerpo4.getData())??>
180 ${{Body4FieldSet.Body4.getData()}
181</#if>
182
183
184<#if (Cuerpo5FieldSet.Cuerpo5FieldSetFieldSet.ladilloRev4.getData())??>
185 <span class="ladillo"> ${Cuerpo5FieldSet.Cuerpo5FieldSetFieldSet.ladilloRev4.getData()}</>
186</#if>
187
188<#if (Cuerpo5FieldSet.Cuerpo5.getData())??>
189${{Body5FieldSet.Body5.getData()}
190</#if>
191 </div>
192 <#if (AudioFieldSet.Audio.getData())?has_content>
193 <div class="bloque-archivos">
194 <h3>Otros contenidos de la noticia</h3>
195
196
197 <div class="bloque-musica">
198
199
200
201
202
203
204
205 <div class="audio">
206 <div class="logo">
207 <img src="/o/unav-principal-theme/custom/legacy/images/audio.gif" height="15px" width="15px">
208 </div>
209 <div class="bloqueDatos">
210 <span class="nombreArchivo">Audio</span>
211 <div>
212 <a href="javascript:void(0);" class="enlace corporativeColor" onclick="mostrar(1); return false">${AudioFieldSet.AudioFieldSetFieldSet.Titulo_audio.getData()}</a>
213 </div>
214 </div>
215 <!-- BLOQUE DEL REPRODUCTOR DE MUSICA QUE SOLO QUEREMOS QUE SE MUESTRE CUANDO PULSEMOS EL ICONO -->
216 <div id="reproductor1" name="reproductor1" class="reproductor" style="display:none">
217 <p>
218 </p><div id="/documents/10174/1899377/130925noticiasdelcampus_inside.mp3">
219 <audio style="width: 200px;" controls="">
220 <source src="/documents/10174/1899377/130925noticiasdelcampus_inside.mp3" type="audio/ogg">
221 <source src="/documents/10174/1899377/130925noticiasdelcampus_inside.mp3" type="audio/mpeg">
222 </audio>
223 </div>
224 <p></p>
225 </div>
226 </div>
227 </div>
228
229
230 </div>
231 </#if>
232
233</div>